Pollution’s second wind
A new model captures the surprising growth of secondary aerosols
Jun 6, 2011 - by Staff
Jun 6, 2011 - by Staff
Bob Henson | 6 May 2011 • The air in the vicinity of Earth’s biggest urban areas includes a wild variety of constituents emitted by cars, factories, trees, and much more. Tracking the fate of such air as it spreads outward is no simple task—especially when pollution from a city can generate new airborne particles (aerosols) as it spreads into the countryside.

Pollution flowing out from Mexico City—one of the largest urban areas on Earth—can trigger aerosol formation for days afterward. (Photo by Carlye Calvin, ©UCAR. This image is freely available for media and nonprofit use.)
NCAR and the University of Paris recently teamed up to produce the most intricate model of organic atmospheric chemistry to date. With this formidable tool in hand, they’ve now produced the first explicit simulation of a vital process: the chemistry by which primary organic aerosols (POA, which are directly emitted) work with other atmospheric components to produce secondary organic aerosols (SOA).
The results jibe with data collected near Mexico City in a 2006 field campaign. They also highlight the importance of SOA themselves. Although climate scientists have focused mainly on incorporating POA, soot, and sulfate aerosols into global models, there could be even more SOA than these other particles in Earth’s atmosphere overall. And whether SOA serve as a net cooling or warming influence on global climate is an open question.
“It’s a very complicated situation,” says NCAR’s Julia Lee-Taylor. She led the successful simulation with GECKO-A (Generator of Explicit Chemistry and Kinetics of Organics in the Atmosphere), working with collaborators that included Sasha Madronich at NCAR and Bernard Aumont in Paris. The team’s progress is outlined in a paper soon to be posted for open review in Atmospheric Chemistry and Physics Discussions. Many other groups are studying this knotty problem, notes Lee-Taylor: “There are several papers published every day.”

Julia Lee-Taylor. (©UCAR. This image is freely available for media and nonprofit use.)
The term ‘complicated’ may be an understatement when it comes to GECKO. Most earlier models typically parameterized SOA rather than allowing them to evolve on their own. In order for GECKO to depict these secondary aerosols explicitly, the model can keep track of more than a million chemical species and several million gas-phase reactions.
The simulation gets rolling with a digitized replica of the air as sampled in Mexico City during the MILAGRO campaign (Megacity Initiative: Local and Global Research Observations). Starting at midnight local time, GECKO tracks this air in a “box model” that heats, cools, brightens, darkens, and responds in other ways to the diurnal cycle. The box also expands over time to incorporate cleaner regional air, which mimics the dilution of actual pollution as it departs an urban area. A week-long run of GECKO takes anywhere from about three to six days on the moderately large server at NCAR’s Atmospheric Chemistry Division.
The trace gases that contribute most to aerosol formation are difficult to sample comprehensively in a field study like MILAGRO, so Lee-Taylor and colleagues drew on a growing body of lab research to flesh out their model. For instance, they built on recent modeling work by NCAR’s Alma Hodzic and others—based on an original lab study by Neil Donahue and Allen Robinson (Carnegie-Mellon University)—to estimate, in the absence of direct observations, how much partially volatile organic vapor would be co-emitted with the POA. Given these calculations, together with vapor pressure estimates for each simulated reaction product, GECKO was able to track condensation and other critical processes.
Perhaps the most striking message from GECKO’s initial runs is that SOA production can thrive long after a pollution plume leaves its urban birthplace. “It’s still making particles for several days downwind,” says Lee-Taylor. The diversity of SOA expands over time as well, with several thousand condensed species contributing to the aerosol in the model.
The impressive prevalence of SOA in Lee-Taylor’s modeling parallels the large numbers reported in other research. After years of uncertainty, recent studies have begun to settle on a global total of organic aerosol somewhere in the neighborhood of 100–200 teragrams per year. It appears that this total may be split roughly equally between POA and SOA, although the true proportion isn’t known.
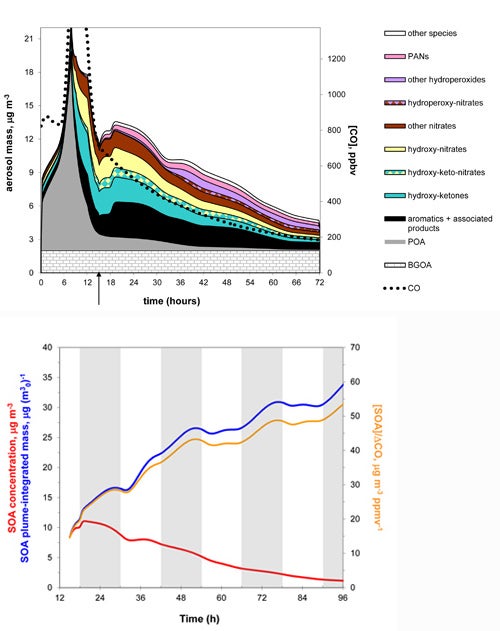
Top: In this output from GECKO, a parcel of Mexico City air that is originally dominated by primary organic aerosol (gray shading) evolves to include a wide range of secondary organic aerosol (other colors) as it flows out from the city over several days. The arrow at bottom indicates the point at which the parcel leaves Mexico City. The SOA formation is especially noticeable on the first afternoon and evening (from 12 to 24 hours on the chart, which corresponds to noon to midnight local time).
Bottom: As the GECKO parcel expands over time to incorporate surrounding air, the concentration of secondary organic aerosols begins to decrease (red line)—yet the total amount of SOA continues to increase (blue line).
(Illustrations courtesy Julia Lee-Taylor.)
“I called it the SOA catastrophe,” says NCAR’s Sasha Madronich, who coauthored the recent study with Lee-Taylor. “Here we have something that’s as abundant as sulfate particles, but we really had no idea where they were coming from.”
Colette Heald (Colorado State University) was one of the first researchers to analyze the discrepancy between modeled and measured estimates of SOA. “The global organic aerosol budget has been a bit of a conundrum for the past few years,” says Heald. “It’s been a challenge to synthesize individual studies into a global picture. I think we’ve narrowed in on how much is out there in the atmosphere. Now we need a combination of more laboratory studies, field studies, and detailed mechanistic modeling to tell us how it was formed.”
Models like GECKO are quickly catching up to the new estimates of global SOA. As recently as 2006, models were predicting as few as 10% of the aerosols that were being observed in the field. “Now we’re able to simulate amounts in the right ballpark,” notes Lee-Taylor.
The implications of those vast amounts of SOA for climate remain murky, though—even in a somewhat literal sense. “Their optical properties aren’t well known. They may be ‘brown’ aerosols,” says Madronich. Most studies to date have assumed that SOA act mainly to scatter radiation rather than absorb it, which means that SOA would have a net cooling effect on climate. Thus, more SOA than previously thought could lead to more cooling. But if SOA are darker than expected, that could add a warming component through absorption of sunlight.
With all this in mind, SOA could prove to be an important wild card in the next assessment from the Intergovernmental Panel on Climate Change. “The 2007 IPCC assessment clearly showed aerosols as the biggest uncertainty in our understanding of climate,” notes Madronich. “That assessment didn’t yet have a good picture of SOA. Now we’re finding that they’re much more abundant than we realized.”
There could be yet another wrinkle. Global climate models do show SOA increasing as cities and pollution grow—but some of that growth is from SOA produced by biogenic trace gases, such as the isoprenes and terpenes emitted by forests. This implies that urban emission plumes might help generate new “pollution” when they drift into greener areas. As Madronich puts it, “The Smoky Mountains might not have been as blue or as smoky as they are now.”
At Pacific Northwest National Laboratory (PNNL), Rahul Zaveri and colleagues found themselves intrigued by this hypothesis. “We went into the chamber and checked it out,” he says. In their lab, the group combined lubricating oil (a proxy for POA) and alpha-pinene (for biogenic emissions) to see how many SOA developed. Then they repeated the experiment without the lubricating oil. According to Zaveri, “We saw no difference in the production of SOA, which surprised us.”
Zaveri is working with other modelers at the University of Colorado Boulder and elsewhere on depicting SOA in regional and global models. He’s cautious about incorporating SOA processes in such models until they’re fully understood. “We have to figure out the exact mechanisms. It’s far more important to get the right answer for the right reason than to get the present-day SOA levels for the wrong reasons.”
Biogenic aerosols are part of GECKO, but they’re only parameterized in the current study, says Lee-Taylor. Her colleagues in Paris are now working on making this component explicit. Another caveat is that GECKO can’t track the chemistry unfolding within aerosols themselves. And even in its present state, GECKO would be far too expensive to include within a global modeling system.
Despite these limits, Zaveri sees GECKO as a welcome step forward. “You can’t measure everything. Right now GECKO is the only model that can provide insight into some of what’s happening. I’m really interested in using GECKO to evaluate our chamber measurements at PNNL and to produce simpler mechanisms that you could begin to put into global models.”