Ozone past and future
What happens next—and what we've avoided
Jan 15, 2010 - by Staff
Jan 15, 2010 - by Staff
3 December 2009 • In the hubbub of the Copenhagen climate summit and its aftermath, a global agreement forged almost a quarter century ago stood quietly in the background, like a modest older sibling. All of the world's nations have now ratified the Montreal Protocol, which sets limits on the chlorine-containing chlorofluorocarbons (CFCs) and bromine-containing halons that were already ravaging the stratospheric ozone layer above Antarctica by the 1980s.
 Ozone has been measured at the British Antarctic Survey’s Halley Station since 1956. (Photo by Bob Pratt, www.photo.antarctica.ac.uk)
Ozone has been measured at the British Antarctic Survey’s Halley Station since 1956. (Photo by Bob Pratt, www.photo.antarctica.ac.uk)
A growing body of research now confirms that the Montreal agreement averted at least one catastrophic form of climate change, even if others still loom. "The Montreal Protocol is a major success story," says William Randel, who directs NCAR's research on atmospheric chemistry.
There's more to the Montreal story. As a side benefit of the protocol, the atmosphere's greenhouse gas burden has been reduced. In fact, by some measures, the protocol's impact on future climate change has been substantially greater than that of the Kyoto Protocol. Some policymakers now see Montreal as an important backstop that deserves to be strengthened as the world debates what might replace Kyoto (see sidebar below).
Meanwhile, the World Meteorological Organization and the United Nations Environment Programme (WMO and UNEP) are now preparing their first major ozone assessment since 2006. The report, due out in early 2011, will be informed by a thorough intercomparison of global models that simulate chemistry and climate. It will also incorporate striking new results from century-scale simulations that track ozone and atmospheric dynamics up to heights of 140 kilometers (87 miles).
 NCAR's David Kinnison and William Randel.
NCAR's David Kinnison and William Randel.
The stratosphere in full
Among the "high-top" models that extend to these altitudes is NCAR's WACCM, the Whole Atmosphere Community Climate Model. It's a hybrid of the center's longtime Community Climate Model, its Thermosphere-Ionosphere-Electrodynamics General Circulation Model, and its Model for Ozone and Related Chemical Tracers. By carefully merging these tools—each developed on separate tracks over decades—WACCM developers have produced a model that can depict the dynamics, chemistry, and radiative processes of the troposphere, stratosphere, mesosphere, and thermosphere, as well as some of the interplay among them.
Models like WACCM are now some of the main research tools for examining stratospheric ozone evolution in the 21st century. Recently, WACCM has been applied to study the projected recovery of stratospheric ozone under various future GHG scenarios, in support of the next WMO/UNEP assessment. A team led by Douglas Kinnison and Rolando Garcia is running WACCM on NCAR's bluefire supercomputer to examine how the presence and absence of ozone affects temperature and circulation. In results now being analyzed, the group has explored what might have happened if atmospheric levels of halogens (CFCs and similar ozone-destroying chemicals) had remained constant from 1960 onward, while other greenhouse gases grew as observed. A separate run looked at the opposite: it included the observed increase in halogens, but held greenhouse gases (GHGs) constant from 1960 onward.
As it turns out, carbon dioxide and other GHGs have a beneficial effect when it comes to stratospheric ozone. WACCM showed that, if GHGs had remained constant since 1960, the eventual global stratospheric ozone recovery would have been delayed by up to 20 years. The fringe benefit of greenhouse emissions occurs because GHGs actually cool the stratosphere, and those lower temperatures slow the stratospheric gas-phase chemistry that depletes ozone.
A world of pain avoided
Other studies are confirming just how severe the damage would have been if ozone-depleting chemicals hadn't been brought under control. NASA's Paul Newman led a study published earlier this year in Atmospheric Chemistry and Physics whose title poses the question answered by the authors: "What would have happened to the ozone layer if chlorofluorocarbons (CFCs) had not been regulated?"
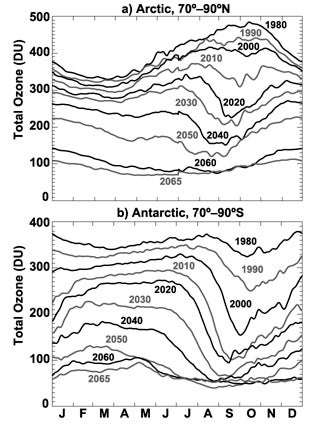 A NASA-led simulation paints a dire picture of how ozone depletion would have unfolded had chlorofluorocarbons not been regulated. Shown above is the steady depletion of total atmospheric ozone (in Dobson units) by calendar month for selected years from 1980 to 2065 above the Arctic and Antarctic. (Image courtesy Atmospheric Chemistry and Physics.)
A NASA-led simulation paints a dire picture of how ozone depletion would have unfolded had chlorofluorocarbons not been regulated. Shown above is the steady depletion of total atmospheric ozone (in Dobson units) by calendar month for selected years from 1980 to 2065 above the Arctic and Antarctic. (Image courtesy Atmospheric Chemistry and Physics.)
The short answer: most of that ozone layer would have vanished. Using a NASA-based model, Newman and colleagues examined the consequences of uncontrolled ozone destruction in stark detail. Among their findings:
The simulation also found eye-opening increases in ultraviolet radiation across populated areas. The UV index for a clear midsummer midday in northern midlatitudes (30° to 50° north, encompassing most of the United States) jumps from an average of 10 at present to more than 30 by 2065. As a result, sunburns would occur in five to seven minutes rather than 15 to 20, and the amount of DNA-damaging ultraviolet light would jump more than 550% over 1980 levels.
"The paper quantifies what many people have thought but never actually calculated," says Newman. "The figure that shows the tripling of the UV index has put a firm stamp on the potential human impact."
 Darryn Waugh (Johns Hopkins University).
Darryn Waugh (Johns Hopkins University).
Putting models in perspective
As shown above, a growing crop of models is now capable of simulating stratospheric ozone decades into the future. Early in the 2000s, scientists decided that these models needed careful evaluation so that their output could be better interpreted for the 2006 WMO/UNEP ozone assessment. Almost a decade of work has unfolded since then under the auspices of SPARC, the Stratospheric Processes And their Role in Climate project within the World Climate Research Programme.
SPARC's CCMVal (Chemistry-Climate Model Validation Activity) is designed to stimulate better modeling. "Our main focus is on assessing what processes the models do well and which ones need improvement," says Darryn Waugh (Johns Hopkins University). Along with Veronika Eyring (German Aerospace Center) and Theodore Shepherd (University of Toronto), Waugh was a member of the steering committee of the CCMVal report, which was finalized in December.
"Usually you never have time to fully understand model results," says NCAR's Kinnison, who joined Martyn Chipperfield (University of Leeds) in writing the report's chemistry chapter. According to Kinnison, CCMVal enabled scientists to thoroughly digest what the models said and how they performed. Strengths and weaknesses were quantified in how the models handled radiation, dynamics, transport, stratospheric chemistry and microphysics, and aspects of the upper troposphere/lower stratosphere region.
There was no single rating for each model in CCMVal—"no model scored high or low on every test, so an overall ranking would be difficult to do even if we wanted to," says Waugh—but each model was assigned a "grade" in each of a variety of categories.
"Obviously modelers don't like it when their model does poorly in reproducing a particular process, and this has created some tension," Waugh notes. However, he adds, in the vast majority of the cases the modelers acknowledge the problem and work to improve the performance of the model. The CCMVal process included two rounds of evaluation, says Waugh, with the second round showing many improvements.
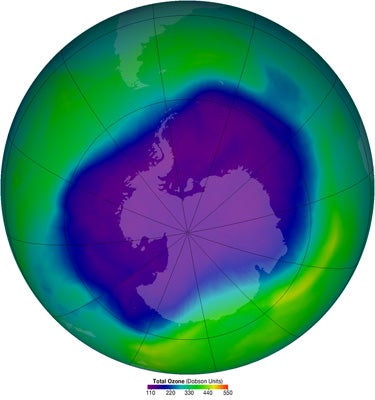 The Antarctic ozone hole of 2006 was the deepest ever observed. Its largest size on a single day-shown in the satellite-derived image above from 24 September 2006-matched the record set on 9 September 2000 of 29.5 million square kilometers (11.4 million square miles). (Image courtesy NASA.)
The Antarctic ozone hole of 2006 was the deepest ever observed. Its largest size on a single day-shown in the satellite-derived image above from 24 September 2006-matched the record set on 9 September 2000 of 29.5 million square kilometers (11.4 million square miles). (Image courtesy NASA.)
How the Montreal Protocol might help turn down the heat
While the Montreal Protocol has long been lauded as a tool of global commitment to cut the risk of ozone destruction, its benefits in reducing global warming weren't fully appreciated until recently. Those benefits are surprisingly large, according to a 2007 study led by Guus Velders (Netherlands Environmental Assessment Agency) and published in the Proceedings of the National Academy of Sciences.
"The climate protection already achieved by the Montreal Protocol alone is far larger than the reduction target of the first commitment period of the Kyoto Protocol," note the authors.
Not only do chlorofluorocarbons facilitate ozone loss, they are also potent and long-lived greenhouse gases. For example, CFC-12 has a lifetime of about 100 years. When their long lifetimes in the atmosphere are taken into account, the total global warming potential per CFC molecule ranges from 5 for methyl bromide to as high as 11,000 for CFC-12 (the value for carbon dioxide is 1). Thankfully, CFCs are far more scant than carbon dioxide, and their emission rates have dropped sharply, although it will be decades before the atmosphere is rid of the CFCs now present.
Velders and colleagues found that if CFCs had not been regulated, their long-term warming impact could have risen to anywhere from 35% to 90% of the value for carbon dioxide as of this year. Later this century, their impact would have soared well past carbon dioxide's, according to the study. That's even taking into account the partial offset caused by the enhanced destruction of ozone, itself a greenhouse gas.
Not only did this study help motivate other "world avoided" studies (including the one led by NASA's Paul Newman, described on page XX), it also spurred interest in using the Montreal Protocol as a more explicit tool for climate protection. This idea was floated by a group of countries at the 21st Meeting of the Parties to the Montreal Protocol, held in November at Port Ghalib, Egypt. One amendment sponsored by Micronesia and Mauritius, and another by Canada, Mexico, and the United States, proposed adding hydrofluorocarbons (HFCs) to the list of gases regulated by the Montreal Protocol.
HFCs are handy, ozone-friendly substitutes for CFCs, but they are also potent greenhouse gases. As such, they are part of the Kyoto Protocol's Clean Development Mechanism, in which developed countries support emission-reducing activities in developing countries. Debate has thus arisen on how the Kyoto and/or Montreal protocols could best tackle the HFC threat without inadvertently promoting a new boost in ozone-depleting substances. The rise of climate-friendly gases and technologies suitable for air conditioning and refrigeration—one of the main growth areas for HFCs—may serve as an end run around these issues.